The CRISPR gene-editing field has ignited a surge of investor enthusiasm from 2023 to 2025, drawing billions into companies pioneering therapies tailored to individual patients’ genetic profiles. This rush stems from CRISPR’s transformation from a basic research tool, discovered in 2012, into a cornerstone of personalized medicine, enabling precise DNA modifications to treat previously intractable diseases. As the market surges past $13 billion in 2025, fueled by regulatory wins and clinical breakthroughs, investors eye a future where one-time gene fixes could redefine healthcare economics.
Continue reading The Investor Gold Rush in Personalized CRISPR MedicineCategory: Multi-Omics & CRISPR
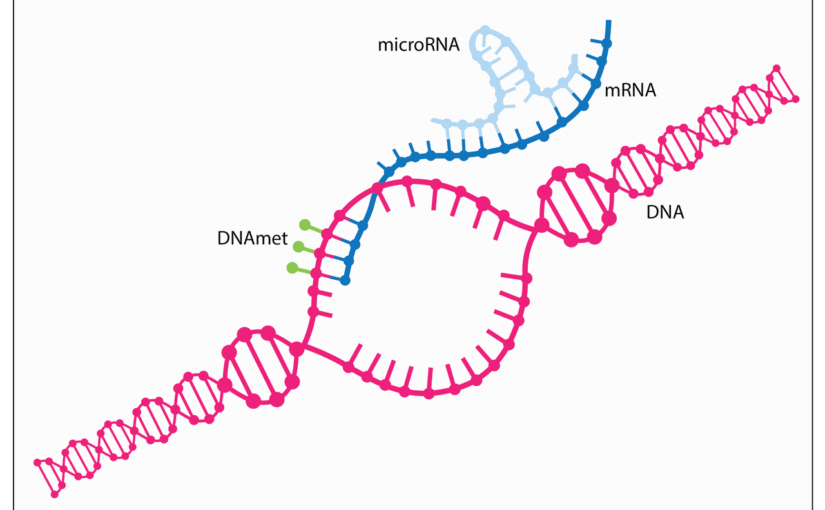
CRISPR Finally Reaches the Mitochondria: A Revolution in Gene Editing
In the cell, a bustling city hums with activity, the nucleus standing as the central library stocked with blueprints dictating eye color, immune responses, and countless other traits. Within the factories powering every operation, mitochondria labor ceaselessly as the core energy generators, converting fuel into adenosine triphosphate, or ATP, the universal currency that sustains cellular functions from muscle contractions to neural signals. These bean-shaped structures, each encased in a double membrane, harbor their own compact genetic archive: a circular loop of mitochondrial DNA, or mtDNA, that encodes 13 vital proteins for ATP production, alongside hundreds more imported from the nucleus. For more than a decade, the groundbreaking gene-editing system CRISPR-Cas9 has reshaped the nuclear library, snipping and replacing faulty genes to combat diseases like sickle cell anemia. Yet these power plants stayed sealed off, their mtDNA impervious to CRISPR’s reach. The culprit lies in CRISPR’s dependence on guide RNAs, delicate strands that bounce harmlessly against the impermeable inner mitochondrial membrane, unable to cross and direct the Cas9 enzyme’s precise cuts. This impenetrable barrier has long shielded mtDNA from editing, leaving scientists grappling with genetic errors that cripple energy supply and doom cells to dysfunction.
Continue reading CRISPR Finally Reaches the Mitochondria: A Revolution in Gene EditingDesigner Dreams or Genetic Nightmare? The Ethical Chaos of Engineering Perfect Babies
In a dimly lit conference room in Beijing last month, a panel of Chinese scientists unveiled a prototype AI system that scans embryos for over 100 genetic traits, promising parents the chance to select for intelligence, athletic prowess, and disease resistance. The announcement, met with cautious applause, highlighted a chilling reality: the race for designer babies has accelerated into a global sprint, where biotechnology blurs the line between healing and engineering human destiny. As one bioethicist put it, “We’re not just editing genes anymore; we’re editing the future of humanity.” This development underscores the urgent ethical tangle in reproductive technology, where innovation races ahead of oversight.
Continue reading Designer Dreams or Genetic Nightmare? The Ethical Chaos of Engineering Perfect BabiesThe Governance Drama Behind CRISPR Food Crops
In the sun-baked fields of Kenya’s Rift Valley, a small plot of golden maize sways under the relentless African sun. This isn’t ordinary corn; it’s a CRISPR-edited variety engineered to withstand prolonged droughts, a beacon of hope amid escalating climate crises. Yet, as farmers eye the resilient stalks, a storm brews far beyond the horizon. In boardrooms and international summits, multinational agribusiness giants push for rapid deployment of such gene-edited crops, citing food security imperatives. But indigenous communities and activists cry foul, accusing these players of biopiracy and eroding seed sovereignty. This tension underscores the global governance drama surrounding CRISPR agriculture, where innovation clashes with ethical imperatives for equitable access and epistemic justice.
Continue reading The Governance Drama Behind CRISPR Food CropsMulti-Omics Goes Mainstream…But Who Owns the Data?
In the fast-evolving world of precision medicine, multi-omics is no longer a niche pursuit. Researchers and clinicians are increasingly layering genomics, proteomics, transcriptomics, and metabolomics to uncover the intricate workings of human biology. They turn raw data into tailored treatments for complex diseases like cancer and Alzheimer’s. This integration promises breakthroughs in clinical trials, where multi-omics profiles can predict patient responses with unprecedented accuracy. It shifts healthcare from one-size-fits-all to truly personalized care.
The revolution feels electric. Yet beneath the promise lies a data deluge that’s reshaping the biotech landscape.
Continue reading Multi-Omics Goes Mainstream…But Who Owns the Data?AI-Created Viruses: A Glimpse Into the Future of Medicine and its Dangers
It sounds like the premise of a futuristic thriller: a machine that can invent viruses from scratch. Yet this is not fiction. Researchers at Stanford University and the Arc Institute have developed artificial intelligence capable of designing viruses that are not only functional but able to reproduce and kill bacteria. The achievement blurs the line between science fiction and scientific reality, offering both dazzling medical promise and unsettling risks.
We now live in an age where algorithms can shape life at its most fundamental biological level. The question is whether humanity can wield this new tool responsibly, or whether we are playing with fire.
Continue reading AI-Created Viruses: A Glimpse Into the Future of Medicine and its DangersCRISPR Breakthrough: Gene-Edited Cells Offer Real Hope for Diabetes Treatment
CRISPR offers remarkable new hope for treating diabetes. Early trials show that gene-edited pancreatic cells can produce insulin in people living with type 1 diabetes, and this could replace a century-old reliance on insulin injections. While massive challenges remain, a wave of clinical research, biotech investment, and cautious optimism is rapidly reshaping our sense of what might become possible in the future for both type 1 and type 2 diabetes patients.
Continue reading CRISPR Breakthrough: Gene-Edited Cells Offer Real Hope for Diabetes TreatmentUnlocking AI’s Power: How Smaller Biotechs Can Speed Up Drug Development in 2025 and Beyond
The landscape of drug development has always been unforgiving, particularly for smaller biotechs. Limited capital, long timelines, and sobering success rates mean that many promising programs never reach patients. On average, it still takes more than a decade and billions of dollars to bring a new therapy to market, and less than 10 percent of drug candidates survive the clinical gauntlet. For resource-constrained startups, these odds are daunting.
Continue reading Unlocking AI’s Power: How Smaller Biotechs Can Speed Up Drug Development in 2025 and BeyondThe $236 Billion Patent Cliff: How 190 Drug Expirations by 2030 Are Sparking a Pharma M&A Frenzy
A second great patent cliff is approaching, and it is a big one, with roughly 190 branded medicines losing exclusivity by 2030, including about 69 blockbusters, putting an estimated 236 to 300 billion dollars in annual sales at risk across the sector by the end of the decade. In this wave, biologics sit at the center, with oncology, immunology, and cardio‑metabolic mainstays facing biosimilar and generic competition, a shift that will cut prices and compress margins even for market leaders. Five of the top ten drug makers could see more than half of revenue exposed, a pressure that is accelerating mergers, acquisitions, and R&D partnerships as companies race to rebuild pipelines and protect future growth. For investors and operators, this creates a dynamic market, where dealmaking, external innovation, and programmatic M&A become core strategies, and where omics and gene editing platforms emerge as prime targets for the next generation of precision therapies.
Continue reading The $236 Billion Patent Cliff: How 190 Drug Expirations by 2030 Are Sparking a Pharma M&A FrenzyPrivate Equity Reshapes Pharma Innovation: AI and Digital Tools Accelerate Drug Discovery and Clinical Trials
Private equity involvement is rapidly increasing in companies deploying artificial intelligence and digital tools to innovate drug discovery and clinical trial pipelines. This trend is reshaping the landscape of pharmaceutical innovation, offering new avenues for efficiency, scalability, and ultimately, better patient outcomes. Recent years have seen significant investor confidence, with global investments in top AI-focused pharma companies totaling billions of dollars and private equity firms playing a pivotal strategic role.
Continue reading Private Equity Reshapes Pharma Innovation: AI and Digital Tools Accelerate Drug Discovery and Clinical Trials